To help healthcare organizations and developers navigate the HTI-1 Final Rule’s compliance requirements, we’ve outlined the estimated hours required to meet each regulation based on the HTI-1 Final Rule’s hours table. Each illustration presents the estimated upper and lower bound hours needed for completing each requirement. The lower bound reflects smoother, more straightforward implementations, while the upper bound accounts for more complex scenarios such as extensive system updates, resource limitations, or intricate integrations. This time range offers flexibility, helping organizations plan more effectively by accounting for their specific size and the complexity of their technology infrastructure.
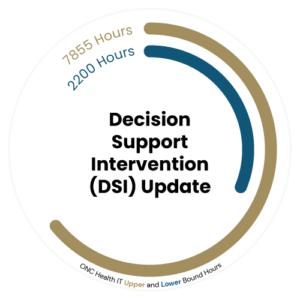
Decision Support Intervention (DSI) Update
Drummond Due Date
December 1, 2024
ONC Deadline
December 31, 2024
The HTI-1 Final Rule introduced significant changes to Clinical Decision Support (CDS) by replacing the (a)(9) requirements with the new Decision Support Intervention (DSI) criterion under (b)(11).
Standardized FHIR End Points Requirement
Drummond Due Date
December 1, 2024
ONC Deadline
December 31, 2024
The HTI-1 Final Rule introduces the requirement for Standardized FHIR (Fast Healthcare Interoperability Resources) Endpoints to enhance data interoperability in healthcare.

This mandates certified EHR developers to implement standardized FHIR APIs, allowing secure and seamless access to patient data across different platforms. By ensuring certified EHR developers adhere to a common set of standards, this requirement promotes real-time data sharing and integration, which is essential for improving care coordination, patient outcomes, and operational efficiency. Compliance with this rule is critical for healthcare providers aiming to meet CMS Promoting Interoperability requirements and to remain eligible for Medicare reimbursements starting in 2025.
Developing, testing, and maintaining these secure and interoperable systems will demand considerable time, resources, and expertise, especially as real-time data integration across platforms can be technically challenging. Given the complexity of this task, it’s better for certified EHR developers to begin working on compliance well in advance. Early preparation allows for thorough testing and refinement of systems, reducing the risk of errors or setbacks as deadlines approach. Starting early also provides more time to allocate resources effectively, address potential security concerns, and adapt to any unforeseen technical hurdles.
Insights Condition and Maintenance of Certification Requirements
Under the Insights Condition and Maintenance of Certification requirements, the rule sets annual reporting obligations for certified EHR developers to ensure ongoing compliance and transparency.

Starting January 1st, 2026, developers must begin collecting data for the entire year, to be reported to the Office of the National Coordinator for Health Information Technology (ONC), detailing key metrics such as system performance, clinical impact, risk management strategies, and the integration of technologies like Predictive Decision Support Interventions (PDSI). To meet this requirement, certified EHR developers will need to implement a critical software update that enables comprehensive data collection, ensuring their reporting software is fully operational throughout 2026.This update is a significant part of the estimated effort included in the Insight Condition and Maintenance of Certification Requirement process.
These reporting requirements will also evolve over time to reflect changing priorities in healthcare technology. In the first year, reports will focus on foundational metrics like interoperability and real-time data sharing, while subsequent years will require more advanced assessments. This phased approach ensures that certified EHR developers remain current, safe, and effective in the rapidly evolving healthcare landscape.
Given the complexity and scope of these requirements, it is critical for certified EHR developers to start working on compliance early. The necessary software updates and data collection capabilities will take time to develop, test, and deploy. Starting early allows for thorough planning, minimizes the risk of last-minute issues, and ensures sufficient time to address any challenges that may arise.
USCDI Version 3 Requirement
Drummond Due Date
December 1, 2025
ONC Deadline
December 31, 2025
The HTI-1 Final Rule mandates the adoption of USCDI Version 3 as the new data standard for certified EHR developers by December 31, 2025.

The shift to USCDI v3 introduces 47 new data elements, updates to vocabulary standards, and changes to data classes, which will impact various certification criteria. Certified EHR developers must ensure their systems can seamlessly exchange and utilize this expanded data set by collaborating with software partners and upgrading systems to meet compliance deadlines. Delaying this work could lead to rushed implementations, increasing the risk of errors, disruptions, or failure to meet certification deadlines. Early action allows developers to gradually introduce updates, address challenges as they arise, and refine their systems to ensure a smooth and compliant transition by the 2025 deadline.
Electronic Case Reporting Requirement
Drummond Due Date
December 1, 2025
ONC Deadline
December 31, 2025
An updated Electronic Case Reporting (eCR) requirement, aimed at improving the timely and accurate exchange of health data for public health purposes has been introduced under the rule.

As a result, EHR developers certified to (f)(5) must support specific clinical cases to public health agencies, ensuring critical health information is shared swiftly during outbreaks or other public health events. This requirement enhances public health surveillance by streamlining the collection and transmission of data directly from healthcare providers to relevant authorities. Compliance with eCR is essential for healthcare organizations to meet federal reporting standards and contribute to more effective public health responses.
Even though the effort required for compliance with this eCR update is considerably lower compared to other requirements, it’s still important to begin working on it early. Early action allows certified EHR developers to identify any potential integration issues, ensure seamless automation, and avoid unexpected delays that could arise from system updates or coordination with public health agencies.
Patient Demographics and Observations Certification Requirement
Drummond Due Date
December 1, 2025
ONC Deadline
December 31, 2025
The HTI-1 Final Rule introduces a revised Patient Demographics and Observations certification requirement, expanding the scope of demographic data that certified EHR developers must capture and manage.

Formerly focused on basic patient demographics, the new requirement—titled “Patient Demographics and Observations”—includes additional data elements from USCDI Version 3, such as social determinants of health, gender identity, and other key attributes. Certified EHR developers must now enable users to record, update, and access this broader range of patient information. This enhancement is designed to provide healthcare providers with a more comprehensive view of the patient, improving personalized care and data accuracy while promoting equitable healthcare delivery.
Starting early on compliance for this requirement is crucial, even though the effort may seem manageable. Early action allows developers to ensure seamless integration of new data elements and test system performance to avoid any potential disruptions or delays. Addressing the changes ahead of time reduces the risk of last-minute technical challenges that could impact data accuracy or user experience. It also ensures that certified EHR developers are fully equipped to support healthcare providers in delivering more personalized and equitable care as soon as the requirements take effect.
Patient Access Restriction Requirement
Drummond Due Date
December 1, 2025
ONC Deadline
December 31, 2025
The HTI-1 Final Rule introduces a new Patient Requested Access Restrictions requirement, empowering patients to have greater control over who can access their health information. Furthermore, certified EHR developers must now support functionality that allows patients to request restrictions on the sharing of their electronic health information (EHI) with certain providers or entities.
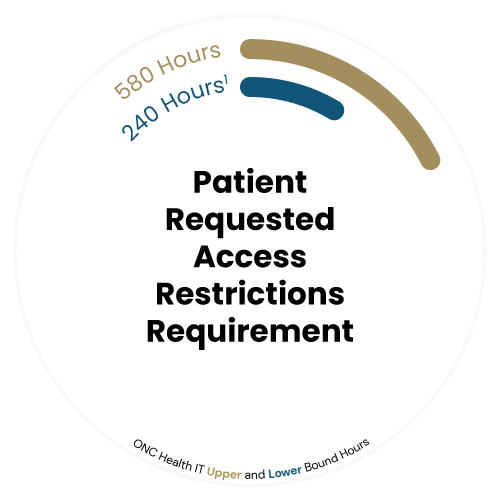
This feature ensures that healthcare organizations can receive patient preferences while maintaining compliance with privacy regulations. By providing patients with this enhanced control, the rule promotes patient autonomy and trust in how their health data is managed and shared, reinforcing the importance of privacy and individualized care.
It is beneficial to start working on compliance for this requirement early, even though the technical effort may appear straightforward. While the rule only mandates the patient-facing request function, EHRs may have additional design considerations relating to the handling of these requests. Early preparation allows developers to thoroughly test and refine the functionality, ensuring that the systems not only meet regulatory standards but also provide a seamless and user-friendly experience for patients. Additionally, starting early gives healthcare organizations more time to train staff and adjust workflows to accommodate this new feature, fostering better patient engagement and trust from the outset.
